

6th Floor, Clinic Bldg.
2799 West Grand Blvd.
Detroit, MI 48201
(313) 916-2964
Specimen Transportation


|
|
| |
    |
 |
 |
|
|
Specimen Submission Requirements
|
| |
Specimen Labeling Requirements
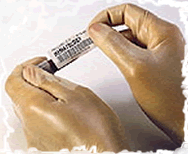
For patient safety, each specimen container must have a label firmly affixed to the container, not the container lid or biohazard specimen transport bag, that contains the following minimum information:
- Patient’s full name (last name, first name);
- Patient’s medical record number;
- Date of specimen collection;
- Time of specimen collection (time sensitive specimens); and
- Specimen description or anatomic site of the specimen (non-blood body fluids,
microbiology, cytology and surgical tissue specimens).
Properly labeled specimen(s) must be accompanied by a requisition (electronic order) containing the following information:
- Patient’s full name (last name, first name);
- Patient’s medical record number;
- Date and time of specimen collection;
- Source or site of specimen (non-blood body fluids, microbiology, cytology,
and surgical tissue specimens);
- Preoperative and postoperative diagnosis and clinical history (cytology
and surgical tissue specimens);
- Test(s) requested;
- Collector identification;
- Patient location;
- Ordering physician name and doctor code (senior staff only); and
- ICD diagnostic code (OPD charges and IPD professional charges).
TThe person that collects a specimen from a patient for laboratory analysis is responsible for establishing the identity of both the patient and the specimen(s) at the time of collection. Patient identification must be verified immediately before any specimen collection procedure. Specimens MUST be labeled in the presence of the patient.
- Patients with ID bands will be identified by comparing their ID band to
the specimen label or requisition.
- Patients who are not required to wear an ID band will be identified by
asking the patient to state their first name, last name and date of birth.
Specimens submitted to the laboratory without the required information will not be accepted for processing. Unlabeled blood and urine specimens will not be accepted for testing and may require recollection. Although extenuating circumstances may exist and some information may be retrievable after specimen receipt by the laboratory, this will require the licensed caregiver, physician or nurse, to come to the laboratory to provide the missing information and sign a Specimen Labeling Resolution form, accepting responsibility for the accuracy of post-collection labeling. Post-collection labeling (rehabilitation) will be permitted under the following circumstances only:
- The test is a Vital Value and delay resulting from recollection could compromise
care;
- Clinical reasons exist for avoiding recollection (requires approval of pathologist/division
head, laboratory supervisor, or designee); or
- The patient is unavailable for recollection (requires approval of pathologist/division
head, laboratory supervisor, or designee).
Test Requisition
Electronic
order entry or paper requisition forms may be used to initiate laboratory tests.
It is the responsibility of individuals performing electronic order entry of
laboratory tests to accurately and completely transcribe the tests ordered by
the physician. A laboratory order must include:
- The patient's
last name, first name, medical record number (MRN)
- Patient
location
- Ordering
physician including secondary provider
- Diagnostic
code(s) for requested tests
- Specimen
collection date and time
- Individual
tests meeting medical necessity or HCFA approved panels, or tests where the
physician has acknowledged medical necessity requirements and reimbursement
limits
- A paper
requisition must accompany every surgical pathology and cytopathology specimen
|
| |
Specimen Submission Requirements for Surgical Pathology and Cytology
For specimens sent to referral laboratories, the referring laboratory must properly follow all requisition, collection and handling specifications of the referral laboratory.
- Specimens must be collected in proper containers. (Refer to sections B and C below)
- Outdated/expired containers may not be used.
- Samples that are improperly labeled or stored may not be processed.
- Specimens may need to be rejected for quality purposes.
HFH Collection and Handling Specifications for Routine Specimens |
A. HFH Special Timing for Collection
- When submitting breast tissue pathology specimens removed for lesions clinically suspicious for malignancy, the cold ischemia time (removal of the tissue from the patient (TOC)) and time placed in fixative (TIF)) must be included in the requisition form. Refer to Breast Tissue Pathology ELUG.
- Muscle Biopsies - the specimen should be submitted to the pathology department immediately and identified as a muscle biopsy to the pathology personnel. Offsite specimens should be delivered to the Henry Ford Hospital Pathology Laboratory as soon as possible. Regardless if the biopsy is done in house or off site, the specimen must arrive by 3:00 p.m. Refer to Muscle and Nerve Biopsy ELUG.
- Nerve Biopsies- In house specimens should be submitted to pathology immediately labeled "fresh nerve biopsy." Offsite specimens should arrive at the Henry Ford Hospital Pathology Laboratory as soon as possible. Refer to Muscle and Nerve Biopsy ELUG.
- Lymph Node Biopsy for Lymphoma Work-up - In house specimens are submitted to pathology immediately labeled "lymph node biopsy for lymphoma work-up." Biopsies from remote sites should arrive at the Henry Ford Hospital Pathology Laboratory as soon as possible. Refer to Lymph Node Biopsy ELUG.
- Kidney Biopsies- In house specimens are handled by Surgical Pathology Staff immediately after removal from patient. Offsite specimens must be delivered the same day as collection by cab, A1 delivery service, or courier and arrive at the Henry Ford Hospital Pathology Laboratory, preferably by 4:00 p.m. Monday - Friday. Refer to Kidney Biopsy ELUG.
- Embryo, Fetus and Stillborn surgical samples require specific handling and paperwork to be submitted along with the samples. Refer to Tier 1: Fetal Demise / Neonatal Demise Procedure for workflow specific to department.
Final Disposition of Stillbirth
HFHS Final Disposition of Fetus |
B. HFH Type of Collection Container and Amount of Specimen to be Collected
- Use appropriate specimen containers according to the size of specimen submitted. The lids of the containers must be appropriately closed to avoid any leaking of body fluids and/or fixatives.
|
C. HFH Types and Amounts of Fixatives
Surgical Pathology
- 10% neutral phosphate-buffered formalin (NBF) is the preferred fixative and should be used for fixation of routine surgical pathology samples. For fixation of specimens in formalin, specimens must be fully submerged with 10% neutral phosphate-buffered formalin to approximate specimen volume of 10:1 or higher, or if not feasible (e.g., large specimens) at least 4:1.
- Special handling and transport of specimens is required for specific tissue types, please refer to ELUG entry for the specific tissue type.
Cytology
|
D. Specimen Labeling and Requisition/Order Requirements
- For patient safety, each specimen container must have a label firmly affixed to the container, not the container lid or biohazard specimen transport bag, that contains the following minimum information:
- Patient’s full name (last name, first name)
- Patient’s medical record number
- Date of specimen collection
- Time of specimen collection
- Specimen description or anatomic site of the specimen
- A combination of the electronic order and paper requisition must contain the following information, if applicable to specimen type:
- Patient’s full name (last name, first name)
- Patient’s medical record number and/or date of birth
- Date & time of specimen collection
- Source or site of specimen
- Preoperative, postoperative diagnosis and clinical history, including last menstrual period, where applicable
- Test(s) requested
- Collector’s identification
- Patient location
- Patient gender or sex
- Authorizing provider’s name and doctor code
- Address of authorizing provider
- ICD diagnostic code
- The person that collects a specimen from a patient for laboratory analysis is responsible for establishing the identity of both the patient and the specimen(s) at the time of collection. Patient identification must be verified immediately before any specimen collection procedure. Specimens MUST be labeled in the presence of the patient.
- Patients with ID bands will be identified by comparing their ID band to the specimen label or requisition.
- Patients who are not required to wear an ID band will be identified by asking the patient to state their first name, last name, and date of birth.
- Specimens submitted to the laboratory without the required information will not be accepted for processing. Unlabeled surgical specimen containers will not be accepted for testing and may require recollection.
- Note: Although extenuating circumstances may exist and some information may be retrievable after specimen receipt by the laboratory, this will require the licensed caregiver, physician or nurse, to provide the missing information and sign a Specimen Labeling Resolution form, accepting responsibility for the accuracy of post-collection labeling.
|
|
| |
|
|
|
 |
 |
 |
 |
 |
Last Modified:  Wednesday, February 26, 2025 3:21 PM
Wednesday, February 26, 2025 3:21 PM
|
|
| |
home | Accreditation/Certification
Information | mailing list | contact webmaster
LUG(Lab User's Guide) Copyright 2022 Henry Ford Health |
|
|
|





