

6th Floor, Clinic Bldg.
2799 West Grand Blvd.
Detroit, MI 48201
(313) 916-2964
Specimen Transportation


|
|
| |
  |
 |
 |
|

|
Coagulation Specimen Collection, Processing, And Transportation
|
| PRC-PALM-SPC-5.51-pro1-prs3: COAGULATION SPECIMEN COLLECTION AND TRANSPORT (Print here) |
Specimen Collection
- Collect specimen in blue top (3.2% sodium citrate) tube. If multiple tests are being drawn, draw coagulation studies before additive-containing tubes such as the EDTA, heparin, or clot activator (SST) tubes. If the coagulation tube is being drawn using a winged collection device with variable tubing length so air in the tubing is not introduced into the blood collection tubes leading to under-filling, draw 1-2 mL into another blue top (3.2% sodium citrate) Vacutainer®, discard, and then collect the specimen. When using the hypodermic needle/syringe, it is important the blood is added to the appropriate volume of anticoagulant within one minute of completion of draw.
- Once obtained, the tube should be gently inverted three or six times end over end inversions; do not over mix as excessive mixing will affect the test result. Insufficient mixing may have a greater effect on specialized hemostasis assays. Citrate tubes must be adequately filled (to the mark noted on the tube if provided) or to not less than 90 % of this total volume. Inadequate filling of the collection tube will lead to inaccurate results.
- The final citrate concentration in the blood should be adjusted in patients who have hematocrit values above 0.55L/L (55%). For hematocrits below 02.0L/L (20%), there are no current data available to support a recommendation for adjusting the citrate concentration.
|
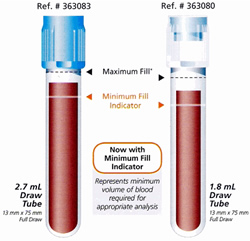 |
| PRC-HFH-COA-5.51-stw3: VISUAL AID OF VOLUME REQUIREMNTS FOR COAGULATION SAMPLES STANDARD WORK |
|
- When drawing the specimen, avoid contaminating the sample with tissue thromboplastin as this may affect results. Venipuncture must be clean with no trauma, and the application of the tourniquet should be limited to 1 minute.
- Collection of the blood through lines that have been previously flushed with heparin should be avoided. If the blood must be drawn through a VAD (vascular access device), possible heparin contamination and specimen dilution should be considered. In this case the line should be flushed with 5 mL of saline and the first 5 mL of blood or six dead space volumes of the VAD discarded.
- Blood should never be transferred from 1 collection tube to another in effort to provide the required fill volume. This is true even if two sodium citrate tubes are combined as this may lead to doubling up of anticoagulant citrate levels and further dilution of the plasma sample.
|
|
Transport of Whole Blood Specimens
- Store and transport whole blood specimens at room temperature. Avoid exposure of whole blood samples to ice or freezing temperatures. Unless otherwise directed, transport the specimen to the laboratory at room temperature within two hours of collection. PT specimens from the Medical Centers may be transported to the laboratory at room temperature for up to 24 hours after collection.
- APTT from the HFHS Medical Centers:If patient is not receiving heparin, specimen may be sent in the native tube at room temperature or in a common cooler for up to 12 hours after collection. If delivery to the laboratory will be delayed more than 12 hours, centrifuge specimen at 1500xg (at least 3000 rpm) for 15 minutes or at a centrifuge speed to consistently produce platelet poor plasma. Transfer platelet poor plasma to a polypropylene tube and cap. Avoid contamination of plasma with platelets from the buffy coat layer. Freeze plasma rapidly. Transport specimen to laboratory on dry ice in a suitable container.
- Specimens for other assays (e.g. thrombin time, protein C, Factor V and other Factors) kept at 2°- 4°C or 18 to 24°, should be centrifuged and tested within four hours from time of specimen collection.
- Specimens for tests other than PT/APTT that are collected at Medical centers will require special processing (please refer to the specific tests page for additional instructions as needed). Centrifuge the tube at 1500 x g (3000 RPM) for 15 minutes to separate the plasma. Transfer the platelet poor plasma to a 12x75 mm polypropylene tube. Avoid contamination of plasma with platelets from the buffy layer. Securely cap the tube. Properly label the tube and freeze rapidly at -20°C or below. Specimens can be frozen up to two weeks at -20° or for six months at -70°. Transport specimen to the laboratory on dry ice in a suitable container. DO NOT POOL SPECIMENS FOR COAGULATION. EACH TUBE SHOULD BE ALIQUOTED INTO AN INDEPENDENT TUBE PRIOR TO FREEZING.
- The number of tests that can be done depends on the combination of tests requested, condition of the sample(s), and potential need for reflexive testing.
- Specimens that are improperly filled (under filled/QNS or overfilled), clotted, hemolyzed, received after prolonged delay, collected in an improper container (wrong tube/anticoagulant), collected with the wrong sodium citrate concentration, or collected in an expired collection tube will be rejected.
- For technical information, contact the Special Coagulation Laboratory at (313) 916-1825.
|
|
|
|
 |
 |
 |
 |
 |
Last Modified:  Monday, March 11, 2024 11:33 AM
Monday, March 11, 2024 11:33 AM
|
|
| |
home | Accreditation/Certification
Information | mailing list | contact webmaster
LUG(Lab User's Guide) Copyright 2022 Henry Ford Health |
|
|
|





